Intracellular targeting and sensing
Cellular entry pathways of nanomaterials influence overall labeling and delivery efficiency. We are interested in understanding and modulating cellular behaviors of CPNs by changing the chemical properties of CPs. By monitoring cellular uptake efficiency and immunostaining against caveolin-1 (a protein found in caveosomes), we found that CPNs use caveolae-mediated endocytosis, which is one of the least destructive endocytosis pathways (Macromol. Biosci., 2013). We also found that a subtle change in the side chain and backbone affects subcellular localization. CPNs fabricated from non-ethylene oxide containing, semi-flexible CPs exhibit high Golgi localization (Chem. Commun., 2013). We synthesized mitochondria-targeting CPNs (Chem. Commun., 2016). Using scanning ion conductance microscopy (SICM), we also discovered that CPNs containing primary amines induce high cell surface morphology changes, which is believed to the cellular response to extracellular materials for uptakes (Macromol. Biosci., 2016).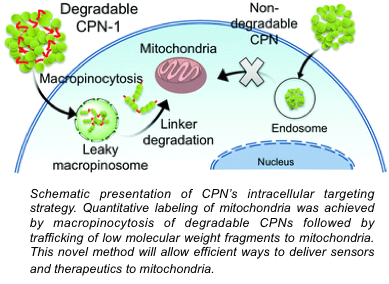
Our goals are to understand how the chemical and physical properties of the CP/gene/drug complex influence cellular behaviors and to demonstrate improved drug efficacy. Our approaches to improve the overall efficiency are to 1) increase cellular entry, 2) deliver the payloads to target organelles, and 3) increase the release of the payloads from the cargo. The preliminary data suggests that further fine-tuning of the chemical and physical properties of CPN/nucleic acids complexes (i.e., polyplexes) can be accomplished by changing backbone lengths, backbone compositions, and types of polyanions. Synthesis of drug and/or hydrogen peroxide sensor conjugated biodegradable CPNs is underway.