Chloroperoxidase
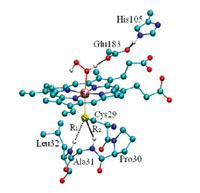
..........................CPO..................................................heme/catalytic residues............................active site model..............................................
Chloroperoxidase (CPO), a protein secreted the marine fungus C. fumago, is a heme-thiolate enzyme with exceptional versatility. Its signature function is to utilize halide ions to halogenate organic substrates, but it is also capable of dehydrogenations (like classical peroxidases), peroxide dismutation (like catalase), and monooxygenation of many organic molecules (like monooxygenases). It is of particular note that CPO-catalyzed oxidation and epoxidation reactions proceed with high regio- and enantiospecificity. Because of these properties, CPO is of interest for potential applications in synthetic chemistry and in the pharmaceutical industry. Our work includes identifying sources of enantiospecifity for CPO’s epoxidation reaction; quantifying the influence of active-site amino acid residues on the formation and reaction of CPO’s active form, Compound I; and parameterizing appropriate molecular mechanical ligated heme models for classical MD simulations.